Instructions For Use PEDIA Anesthesia Balloons

Please Read All Descriptions, Indications and Warnings Before Use
DESCRIPTION
The pediatric device for induction of anesthesia (PeDIA) is an alternative to a face mask for the inhalation induction of anesthesia and is intended for the delivery of nitrous and/or anesthetic gases to children age three years and older. It is intended to be used prior to IV insertion, LMA/endotracheal intubation, and/or conversion to a standard mask induction.
Please Read All Descriptions, Indications and Warnings Before Use
Instructions for the anesthesia professionals:
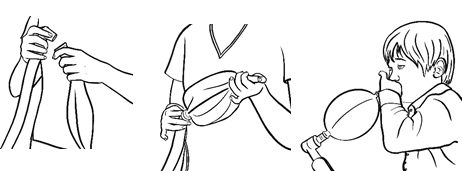
- Attach the universal connector of the PeDIA to the anesthesia breathing circuit as you would the anesthesia mask.
- Turn the APL valve (pop-off) to minimum.
- Obstruct the whistle/mouthpiece using your thumb/finger to trap gases inside balloon.
- Turn up flows of oxygen and nitrous oxide sufficient to inflate the PeDIA.
- Hand the child the inflated PeDIA Balloon.
- Be sure the child seals their lips around the mouthpiece.
- Be sure child inhales and exhales only through the mouth, not the nose.
- As the child exhales and inhales, observe the system is working properly.
- PIPs remain low
- Balloon slightly inflates and deflates.
- After 2-3 breaths, dial-in the volatile anesthetic of choice.*
- When the child falls asleep or is too sleepy to hold a proper seal lips around whistle, take the PeDIA from the child and lay the child supine.
- Detach PeDIA from the anesthesia circuit using a gentle pull.
- Attach the anesthesia mask to the breathing circuit.**
- Support patient respiration with mask-ventilation and continue induction/intubation.
- Discard the PeDIA per facility protocol.
*Noncombustible anesthetic agents should be used with gas flows and anesthetic concentrations determined by the anesthesia provider.
**Before switching to a mask, the practitioner may elect to vent the gases into the circuit.
Instructions: Pediatric Device for Induction of Anesthesia Please read all instructions before use.
WARNINGS:
Warnings:
| Consult instructions for use | |
| Single use; disposable | |
| Keep dry | |
| Caution | |
| No components of the PeDIA are made of latex | |
| Disposables delivered are non-sterile | |
| DO NOT send any portion of the device home with the child | |
| DO NOT use if the device is damaged | |
| DO NOT use if the packaging is damaged | |
| DO NOT use with combustible volatile anesthetics | |
| DO NOT sterilize the device | |
| DO NOT reuse the device or share between patients | |
| DO NOT separate the body of the device from the mouthpiece | |
| DO NOT use with children who cannot or will not follow directions |
| Restricted to sale by or on the order of a physician | |
| Manufacturer of Record | |
| Date of manufactured unit | |
| Catalog Number | |
| Lot Number | |
| Do not use if packaging is open or damaged | |
| Do perform an anesthesia machine check per ASA guidelines to include the scavenger system | |
| Do instruct the child on the proper use of the device | |
| Do inspect the device for signs of damage | |
| Do set and maintain the Adjustable Pressure Limiting Valve (APL or pop-off) to minimum |
![]() Discontinue use with signs of increased pressure within the PeDIA such as:
Discontinue use with signs of increased pressure within the PeDIA such as:
• A taut balloon or increase Peak Inspiratory Pressures (PIP).
• Any signs of patient distress
• The patient cannot or will not follow directions
